Abstract
Chimeric antigen receptor T cells targeting CD30 (CD30.CAR-T) have shown high response rates, including some durable remissions, in patients with relapsed/refractory (r/r) classical Hodgkin lymphoma (HL) (Ramos et al., JCO 2020). However, some patients are non-responders or eventually relapse after therapy. Because CD30 expression is retained in relapsing tumors, recurrence may be due to the insufficient persistence of CAR-Ts within the highly immunosuppressive tumor microenvironment of HL. We therefore reasoned that with enhanced trafficking to the tumor site, CD30.CAR-Ts would have increased opportunities to eliminate tumors before inhibitory mechanisms become predominant. This is especially relevant for HL, where Hodgkin Reed Sternberg (HRS) cells produce CCL17 (thymus and activation-regulated chemokine) to create a physical and inhibitory barrier to cytotoxic T cells. We have previously shown that CD30.CAR-Ts co-expressing the cognate receptor for CCL17, CCR4 (CCR4.CD30.CAR-Ts), have improved tumor homing and anti-lymphoma activity compared with CD30.CAR-Ts that do not express CCR4 (Di Stasi et al., Blood 2009). CCR4.CD30.CAR-Ts should also be more effective in CD30+ cutaneous T-cell lymphomas (CTCL) due to enhanced trafficking to the skin. We present the preliminary results of a clinical trial assessing the safety (primary objective) of this novel strategy and its efficacy compared to CD30.CAR-Ts lacking CCR4 in patients with r/r HL and CD30+ CTCL (NCT03602157).
CCR4.CD30.CAR-Ts are infused in patients in a dose escalation fashion (DL1=2x10 7 CAR-Ts/m 2, DL3=5x10 7 CAR-Ts/m 2, DL5=1x10 8 CAR-Ts/m 2,). To provide definitive conclusions on the role of CAR-T tumor homing, after completion of each dose level, patients receive the dose of CCR4.CD30.CAR-Ts established to be safe in the prior DL, combined with a fixed dose of CD30.CAR-Ts (1x10 8 CAR-Ts/m 2) (DL2, DL4, DL6). All patients receive lymphodepletion with 3 days of bendamustine 70 mg/m 2 and fludarabine 30 mg/m 2. Key inclusion criteria are age ≥ 18 years and r/r HL or CTCL having failed ≥2 prior therapies.
At the time of data cut off (8/1/2021), 6 patients were treated on DL1, 3 patients on DL2, and 3 patients on DL3. The median age is 43.5 (range 27-75) with a median of 5.5 prior lines of therapy (range 2-10). Ten patients had HL and 2 patients had CTCL. All patients had received prior brentuximab vedotin. Eleven patients received prior checkpoint inhibitors, 9 had prior autologous stem cell transplant, and 5 had prior allogeneic stem cell transplant.
The treatment was well tolerated with no dose limiting toxicities observed. Two patients had grade 2 cytokine release syndrome (CRS) which resolved with tocilizumab, and 1 had self-limiting grade 1 CRS. None of the treated patients developed immune effector cell-associated neurotoxicity syndrome.
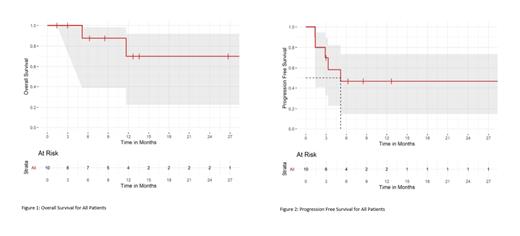
All of the 8 patients with HL who have had disease assessment have responded with 6 complete responses (CR) (75%) and 2 partial responses (PR). Five patients are in remission to date, with one patient still in CR at 2.5 years post treatment. Two patients with HL have responses pending at time of data cut off. Among the 2 patients with CTCL, 1 patient had stable disease and went on to receive alternative therapy and 1 patient had progressive disease. At a median follow up of 12.7 months, the median progression free survival (PFS) for all 10 evaluable patients is 5.2 months and the median PFS for HL patients has not been reached. The median overall survival for all patients has not been reached.
Plasma CCL17, a biomarker of disease response for HL, was reduced by 83±15% by week 2 post infusion in patients treated with CCR4.CD30.CAR-Ts as compared to 52±38% in patients on our previous trial that had received CD30.CAR-Ts lacking CCR4 (p=0.02). In a HL patient on DL1 biopsied 3 weeks post infusion, we found markedly enriched CAR-T signals at the tumor site (14.4 x10 5 copies/ug of DNA) as compared to the signals found at the same time point in the peripheral blood (4.3 x10 5 copies/ug of DNA).
Our data confirm the safety of CCR4.CD30.CAR-Ts as well as their promising efficacy in patients with r/r HL. Interestingly, responses are already seen at the lowest dose level, suggesting that early tumor homing driven by CCR4 may allow more fitted cells to better exploit their antitumor potential. Our data serve as a proof of concept for future modifications of CAR-T cells to improve their localization to disease sites.
Grover: Kite: Other: Advisory Board; Tessa: Consultancy; ADC: Other: Advisory Board; Novartis: Consultancy; Genentech: Research Funding. Morrison: Vesselon: Consultancy. Dittus: BeiGene: Other: Advisory Board; Seattle Genetics: Research Funding; AstraZeneca: Research Funding; Genentech: Research Funding. Dotti: Tessa: Patents & Royalties: Approach for CD30.CAR-T Cells for Hodgkin Lymphoma. Serody: Tessa: Patents & Royalties: Approach for CD30.CAR-T Cells for Hodgkin Lymphoma. Savoldo: Tessa: Patents & Royalties: Approach for CD30.CAR-T Cells for Hodgkin Lymphoma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal